Abstract
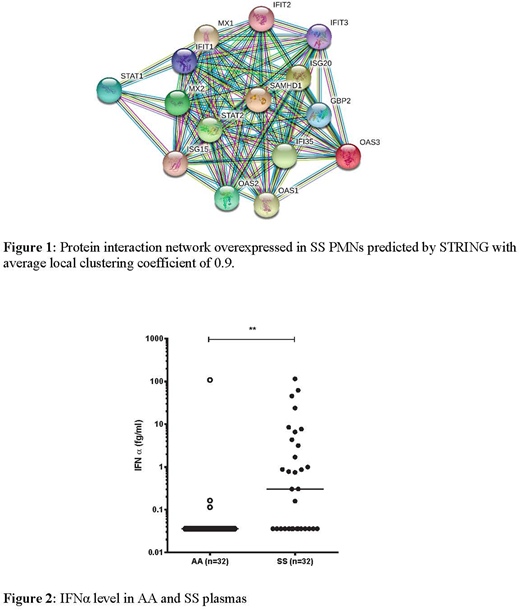
Although sickle cell disease (SCD) is a red cell disorder, many cell types, including endothelial cells and polymorphonuclear neutrophils (PMNs), contribute to its pathophysiology. In particular, activated PMNs have been implicated to play an important role in the initiation and propagation of vaso-occlusive events in SCD. Activated PMNs engage in a complex process of abnormal interactions with activated endothelial cells, platelets and circulating erythrocytes contributing to endothelial injury and decreased blood flow. In the present study, global proteomic analysis was performed using label-free mass spectrometry of PMNs from 4 SCD patients (SS) in steady state and from 4 control subjects (AA). We identified a total of 4,534 proteins both in AA and SS PMNs with 3,080 of these proteins identified in at least three samples for each condition. 50 proteins were significantly over-expressed in SS PMNs compared to AA PMNs (ratio > 1.4). STRING employed to monitor potential interaction between the overexpressed proteins showed that the main interactive clusters consist of STAT1 and STAT2, OAS 1, 2 and 3, and many Interferon Signaling Proteins i.e. IFIT1, IFIT2, IFIT 3, ISG15, ISG20, GBP2, IFI35, MX1 and MX2, TLR8 proteins (Fig. 1). This finding implies a strong activation of the type I interferon (IFN) signaling pathway in the SS PMNs (between 10 and 100-fold increase in SS vs AA). In addition, 33 proteins showed significantly lower expression in SS PMNs compared to AA PMNs. Among these were L-selectin (CD62L) and IL-17 receptor A (IL17RA) (p = 0.01). These findings are consistent with previously described phenotypes of aged neutrophils and acute inflammatory responses in SCD.
Similar proteomic analysis performed on PMNs from SS patients treated with hydroxycarbamide (HC, n=4) showed that 14 proteins had significantly lower expression compared to untreated-SS patients (ratio <0.7). Interestingly, HC restored a normal expression pattern for most of the interferon signaling proteins.
Type I IFNs represent the major effector cytokines of the host immune response against viruses and other intracellular pathogens. These cytokines are produced via activation of STAT1 and of pattern recognition receptors, including the Toll-like receptor signaling network. To determine if type I IFN-α could be detected at the protein level in the plasma of SS patients, we used the novel digital-ELISA technology (SIMOA, Quanterix) developed by Wilson et al (J Lab Autom, 2016). Interestingly, we found an increased level of INFα in plasma from SS patients compared to AA (n=32) (p<0.001) and it is noteworthy that while 50% of SS patients have similar level of INFα compare to AA individuals the other 50% exhibit 10 to 1,000-fold increased levels (Fig. 2).
In summary, our novel proteomic analysis documents a high level expression of interferon signaling proteins, STAT1 and TLR8 in the proteome of neutrophils from SS patients and strongly suggests autoimmune or auto-inflammatory phenomena at basal state in SCD. Our results provide strong support for an important role for the innate immune system in the pathophysiology of SCD. Future studies will help determine the relationship between the plasmatic level of IFN-α and clinical complications and will establish if interferon signaling proteins and IFN-α could represent new therapeutic targets in SCD.
Hermand-Tournamille:Imara: Research Funding. Le Va Kim:Imara: Research Funding. Koehl:Imara: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal